About EURIDICE
EURIDICE stands for European Interoperability Specifications for Digital Solutions in Healthcare. We are dedicated to developing and promoting standardized specifications to enhance interoperability among digital healthcare solutions across Europe.
Why EURIDICE?
The European Health Data Space (EHDS) needs technical specifications that are robust, interoperable, and thoroughly testable. EURIDICE is a joint HL7 Europe and IHE-Europe initiative, focused on creating well-architected and implementable specifications both organizations propose for use in establishing the EHDS.
The aim is to have these specifications offered to the European Commission for being referenced in the EHDS Implementing Acts. In addition EURIDICE intends to support the implementation of the EHDS in all EU member states through proven implementation, validation, and testing methodologies.
It takes the best of both IHE and HL7 organizations, integrating parts that are already available with new specifications developed specifically to support the requirements of the EHDS. EURIDICE turns a specification idea, whether from a project, national initiative, or consortium, into a strong and jointly endorsed artifact through coordinated European testing and listing. For this, EURIDICE:
- Creates technical specifications which support the requirements of the EHDS regulation
- Combines HL7® FHIR®’s agility with IHE’s methodology and testability approach
- Provides a central reference list of community-validated, technical artefact
- Facilitates alignment with EU-funded projects, national priorities, and Standards Developing Organization (SDO) processes
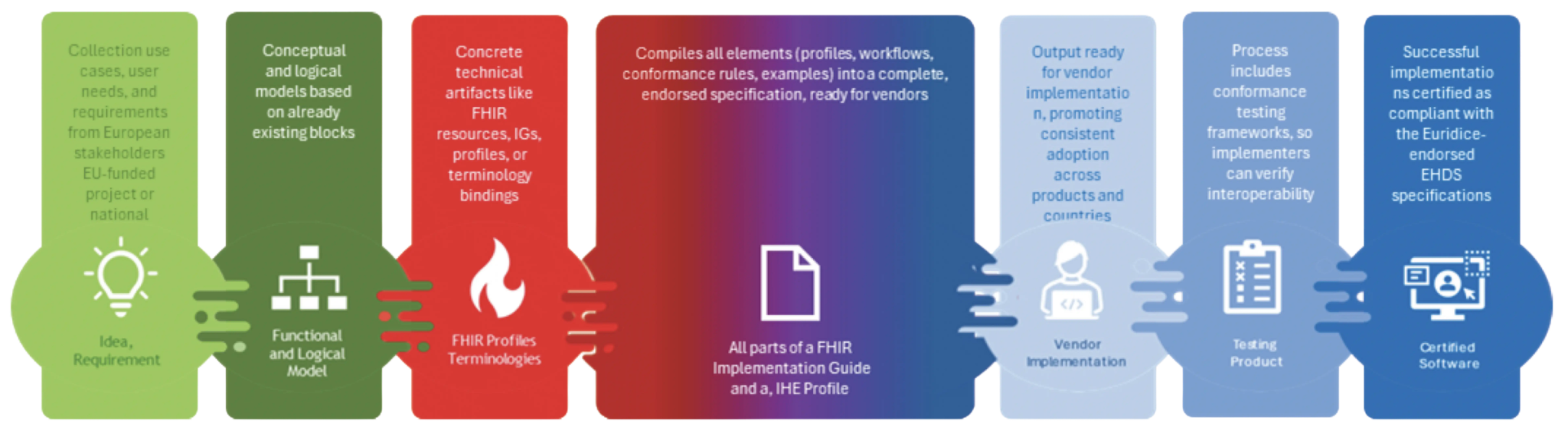
How it works
Every proposal that enters EURIDICE becomes a joint specification developed by IHE-Europe and HL7 Europe together. To make it strong and usable across Europe, we move it forward in three ways at the same time:
- IHE Methodology – the proposal is shaped by reusing the main concepts of the IHE Methodology and – as applicable – IHE Profiles, contributing to their development and evolution.
- EHDS specification mapping – it is added to the EURIDICE list and tested in EHDS Projectathon events to prove it works in practice.
- HL7 Community – it goes through the HL7 Europe’s process, engaging the HL7 Community, becoming a European formal HL7 FHIR Implementation Guide.
By combining these three tracks, EURIDICE produces jointly endorsed, EHDS-ready specifications that are trusted, testable, tested, and recognised across Europe.
Outcomes
- Robust technical artifacts endorsed by both HL7 Europe and IHE-Europe
- Testing in Projectathon
- Considered for EHDS Implementing Acts
- Clear references for EU Member States and implementers
Benefits
- For Member States: ready-made, reliable specifications they can use right away
- For EU-funded projects: a clear route to turn prototypes into widely adopted solutions
- For vendors and implementers: clear, consistent technical requirements they can trust
